I frequently have a sense that mid-century America thought about risk-taking and innovation very differently than we do today. Big institutions in America seemed willing to take big risks on the chance that they’d have big successes in a way that’s unthinkable today.
Like, it’s hard to imagine an equivalent to the Interstate Highway Program, the National Space Program, or even Bell Labs being started today. It seems guaranteed they’d be caught up in politics, laden with additional pork, and ultimately whittled down to something far less ambitious way before they got out of the gate.
This narrowing of ambition seems to be almost universal across big American organizations and industries, with only a few exceptions. While we still see innovation from startups, big companies seem content to rest on their laurels and buy or license innovations from others.
As you might imagine, I’ve been thinking about this recently especially in the context of pharmaceuticals, my own industry. These days, big pharma tends to wait for startups to make and de-risk a scientific innovation, then license or “co-develop” it. Big pharma’s innovations today tend to be much more on the marketing, distribution, and regulatory side.

I recently came across an interesting story in the vein of narrowed ambition regarding the development of omeprazole and its sequel esomeprazole, which you might know better as Prilosec and Nexium. Now, I’d argue that omeprazole is one of the most important drugs in the healthcare system. It is the original proton pump inhibitor and still the eighth most commonly prescribed drug in the US healthcare system. Its effectiveness at treating acid reflux and peptic ulcers means that pretty much everyone ends up using it sooner or later.
As I’ll discuss later, I feel very differently about esomeprazole, which (spoiler alert!), I think is a shameless money-grab that only exists to fill the pockets of big pharma. Its very existence embodies the narrowed ambition and pursuit of profits over science of modern big pharma. But more on that later. First, omeprazole.
The complexity of the saga of omeprazole’s development fits its importance. Omeprazole was very difficult and expensive to develop. AstraZeneca, the developer of omeprazole, started researching gastric acid inhibition in the late 1960s
1. They were successful in rats, but could not find a compound that worked in humans.
In the early 70s, they restarted their search, this time with dogs instead of rats. They ended up finding a compound which worked in dogs but was toxic to humans, so they had to modify it to make it less toxic. Unfortunately, that then made the compound too similar to a Hungarian anti-tuberculosis drug, so AstraZeneca’s scientists focused their work on a metabolite of the compound instead that was not covered by the patent. Fortunately, this metabolite turned out to be even better at gastric acid inhibition than the original compound. It was dubbed timoprazole.
Unfortunately, again, there were toxicological issues. Long-term toxicological studies indicated that timoprazole caused thyroid enlargement. So, it needed to be modified again to avoid the thyroid issues. This time around, the AstraZeneca researchers ended up with a compound they called picoprazole. They almost ran into another toxicological issue after this, when tests of picoprazole seemed to cause necrotizing vasculitis in dogs. But, after further research, this necrotizing vasculitis was discovered that this was actually a side effect of the deworming that the lab dogs went through, so picoprazole was deemed probably safe.
While these toxicological studies were going on, other AstraZeneca scientists were still looking at timoprazole. Research on acid-secreting glands of guinea pigs (later mice and then humans) revealed that timoprazole did not just affect the stomach, but the thyroid as well, tying into the toxicological studies from before. They realized that timoprazole inhibited the H+K+-ATPase proton pump that was the final step of gastric acid secretion
2. They combined their toxicological knowledge from before with their new discovery to come up with a compound that would accumulate in the parietal cells. This was omeprazole.
Omeprazole entered human trials in 1982, a full decade and a half after studies initially began. But, this was not the end of the story. After human trials began, yet another long-term toxicological study showed that high-dose omeprazole caused endocrine tumors in the stomach of rats. So human trials were halted in 1984. Eventually, they figured out that this was not actually a direct result of omeprazole, but only of halting gastric acid secretion, because the same thing happened in rats who received high doses of other gastric acid inhibitors and rats who were surgically altered to have no gastric acid secretion. So, omeprazole was allowed to resume human studies.
Finally, following a comparative study in which omeprazole was put head-to-head against ranitidine, an H2 receptor antagonist (as opposed to omeprazole, which is a proton pump inhibitor), in acid reflux patients and had twice as high healing rates, omeprazole was approved in 1988 in Europe, and in 1989 in the US. From 1988 to 2001, when it went off patent, it generated $26 billion in revenue, making it one of the top selling drugs of all time.
So, that was the story of omeprazole. From conception to release, it took two decades and untold amounts of effort and money. There were at least 4 separate times where it looked like AstraZeneca’s plan of coming up with a gastric acid inhibitor would come to an end, and, at any of those points, the leadership of AstraZeneca could have very easily cut their losses. But they didn’t, and they reaped huge rewards for their monumental efforts. And the public did too, given that they were able to get access to a very effective treatment for heartburn and ulcers.
Now, let’s compare that to the story of esomeprazole, which you might know better as Nexium. Esomeprazole is pretty much AstraZeneca’s sequel to omeprazole. Specifically, it’s the one of the optical isomers of omeprazole. Its story is a lot less impressive than omeprazole. But, before we can get into that, we’ll need some background on what an optical isomer is first.
An optical isomer is another name for an enantiomer of a drug, or a “handed” version of a drug. For those of you who don’t remember chemistry class (or never took it), handedness is a property of some molecules in which they can exist in two possible configurations, right or left. These are similar to right and left hands, in that they can’t be superimposed on each other by rotating. Or, if you want to think about it another way, there’s no way to make a left glove fit a right hand, no matter how much you flip the glove around.
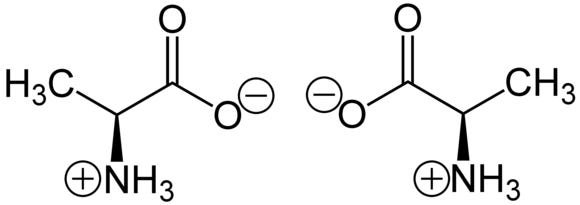
In the body, enzymes, which make reactions happen on biologically relevant timescales, are traditionally understood to work on a lock and key method. As you might imagine, a right-handed key will fit differently to a lock than a left-handed key. So, a pharmaceutical will fit an enzyme more or less well depending on whether it’s the right-handed version of the pharmaceutical or the left-handed version.
That’s where a drug like esomeprazole comes in. When you manufacture a drug with a stereocenter (the part of a molecule that the handedness centers around), you’ll naturally manufacture a “racemic mixture”, meaning an equal amount of left-handed and right-handed drug. You have to apply an extra separation step to get only left-handed or only right-handed drugs.
So, if you’re a pharmaceutical company, you might think, “I bet one of those enantiomers [the technical term for just the right or left hand of a drug] is more effective than the others. Let’s apply a separation step. Then, whatever we’re left with will be the equivalent of a super effective form of the drug.”
You can then argue to doctors, patient, investors, and most importantly, the patent office, that your drug is, somehow, a super effective version of the drug, thus clearing that all important novel and non-obvious bar
3. This is in a nutshell what AstraZeneca did, which I’ll get to in a moment.
You see, the story that AstraZeneca likes to tell about the invention of esomeprazole is that they were looking for a version of omeprazole that would benefit the 2-4% of the population that metabolizes omeprazole poorly because of a mutation in their liver enzymes. These people still benefit from omeprazole, but just have to take more of it more often.
While this has literally no drawback other than inconvenience on the part of the poor metabolizers, AstraZeneca thought it’d be nice if they had a more bioavailable form of omeprazole. As it turns out, after a long period of searching, the left-handed version of omeprazole is the best way to increase bioavailability (so you get more inhibition per each mg). They then figured out how to use chromatography to efficiently separate the isomers, and, voila, had the sequel to their drug.
To prove that this was a more effective drug, they compared 20 mg omeprazole vs. 40 mg esomeprazole in an 8 week trial of acid reflux. Unsurprisingly, 40 mg esomeprazole was more effective at treating acid reflux. They were unfortunately so pressed for time that they didn’t get the chance to compare 40 mg omeprazole vs. 40 mg esomeprazole, and jumped straight into getting the drug approved.
Esomeprazole was approved in 2000 and given the name Nexium. AstraZeneca immediately got to marketing it as an improved version of omeprazole, while simultaneously trying to sue anyone who was gearing up to make a generic version of omeprazole by claiming they were infringing on a manufacturing patent, regardless of whether or not that was true.
These strategies worked, as AstraZeneca was not only able to eke out 6 months additional exclusivity for omeprazole/Prilosec, a drug that made $71 million a week, but they also ended up making $50+ billion from Nexium until it went off patent in 2015. Or, in other words, twice the amount they made from omeprazole with way less development work.
All of this wouldn’t have been so bad if Nexium was a good drug. But it’s not. It’s really just a more potent form of omeprazole, and it’s not even that much more potent. This is why AstraZeneca had to stack the deck and compare 40 mg of esomeprazole vs. 20 mg of omeprazole. When one compares 40 mg to 40 mg, they’re much more similar in potency.
And, I can’t reiterate this enough, there’s literally nothing stopping anyone from just taking more omeprazole if they feel like it’s not working. It’s basically impossible to overdose on it, unless you take it for a long enough time that your stomach acid stops doing its job (which is the same problem you’d have if you took esomeprazole for a long time).
When you look at the numbers, you understand why AstraZeneca did what they did. They got to benefit from all of the good parts about drug repurposing that I’ve taken advantage of in my own repurposing effort: the previously existing manufacturing
4, the safety data, the clues about efficacy. But, at the same time, they got to pretend they weren’t doing drug repurposing. So, doctors didn’t think they could still prescribe omeprazole for this condition, and patients didn’t think they could still take it. Instead, doctors and patients thought they needed AstraZeneca’s new, hot drug.
But, still, what a narrowing of ambition. Creating the most effective gastric acid inhibitor from scratch is a good goal for a drug. It might not be curing cancer, but it’s a good goal. Persevering through so many potentially program-ending events is inspiring to any pharmaceutical company, big or small. Finally, getting omeprazole out there and making so much money off of it feels like a worthy reward.
Then, turning around and using patent lawsuits, bullshit studies, and aggressive marketing to make almost twice as much as you did when you used good science? Well, that’s just depressing.
I have hopes that America’s pharmaceutical industry is turning a corner. Next generation blockbusters like Keytruda and Humira are legitimately cool, even if they’re plagued by their own patent shenanigans. Even drugs like semaglutide, which seem straightforward enough, have some very impressive chemistry in their development stories. This isn’t even to mention the crop of interesting startups that are trying to make their own mark in the pharmaceutical world, both big (Moderna) and small (Highway Pharm!).
Hopefully, we’ll look back at the era that produced Nexium as an anomaly, a time in which we ran out of good science and used bullshit as a substitute. I just wish that there was a better moral to it than, “Don’t do bad science or else you’ll make $50+ billion in sales.”
This account of the invention of omeprazole is taken from AstraZeneca’s own published account in Nature Drug Discovery. There are a ton of interesting chemistry details in there if you care to read their actual account.
I don’t actually understand this leap in logic, but it’s what they say they realized. The closest I can get to understanding it is that Hashimoto thyroiditis, a thyroid autoimmune disease, often also involves anti-parietal cell antibodies, which are the acid-secreting glands in the stomach. I don’t know how you jump from that to “anti-thyroid antibodies necessarily means that it inhibits the final step in gastric acid production in the parietal glands”, as that almost seems like a logical fallacy. But hey, I’m not the guy that invented omeprazole, so what do I know
I’ve talked about this a few times.
People who have never had to manufacture things are often shocked at how difficult and expensive manufacturing things is. You can see this when software companies move into making devices, and all of a sudden they have to worry about stuff like supply chains and logistics.
Manufacturing pharmaceuticals is especially tough. For a new chemical entity, you have to figure out how to: make a new drug, scale it up from something that one PhD working in a lab can do to something that 1000 workers in a factory can do, keep it stable for up to two years, and get all the materials in the right places at the right time. All the while the FDA is breathing down your neck and ready to shut you down if you mess up a step. Knowing a drug is already manufacturable is a big deal.