I’m going to do something a little different for this blog post. Normally, I like to set up questions and answers in the same blog post. But, as I was trying to do that for this blog post, I realized that it was going to be an absolute monster if I tried to do that. So, I decided I’ll set up the questions here, and try to answer them in two separate blog posts.
Alright, enough with the intro. Onto the business at hand: trying to figure out the mysteries of GLP-1 agonists.
Now, let me start off with my general feeling on GLP-1 agonists: GLP-1 agonists, like semaglutide (Ozempic/Wegovy), and GLP-1/GIP dual agonists, like tirzepatide (Mounjaro/Zepbound), are undoubtedly miracle drugs in many ways. I am confident saying that. They consistently cause people to lose weight, they decrease all-cause and cardiovascular specific mortality, they’re very safe, and they are easy to use. They also happen to be reasonably easy to manufacture, as modern pharmaceuticals go, and they are surprisingly easy to invent, judging by the number of GLP-1 agonists that are coming on the market.
The combination of all these factors means that GLP-1 agonists and related drugs are serious technological and scientific achievements. Safe, sustainable, easy weight loss has been one of the biggest goals for the health industry at large1 for at least 70 years, and these drugs are the closest we, as a species, have gotten to achieving that goal. The billions of dollars that Novo Nordisk, Eli Lilly, and startups like Viking have gotten for these drugs is well-deserved, as they represent real innovation in pharma2.
So, this is my base for all of this stuff. They’re good drugs. If you were hoping that the mysteries of GLP-1 agonists had something to do with the possibilities that they weren’t good drugs, then you may be disappointed right now. My apologies.
But, given that they are really good drugs (and, you know, multibillion dollar efforts in both costs and revenue), that means that anything related to making them work better is a really interesting mystery. So, that’s what I’d like to explore today: mysteries around how we can make GLP-1 agonists work better. Here goes:
1. Why do GLP-1/GIP dual agonists work better than GLP-1 agonists?
Tirzepatide (a.k.a. Mounajro/Zepbound), as a GLP-1/GIP dual agonist, is often sold as a sequel to GLP-1 agonists like semaglutide. In a way, it is, at least in terms of greater weight loss, possibly fewer side effects, and seemingly more sustained weight loss.
But GIP agonism is really weird, and it makes it actually pretty hard to understand if tirzepatide is a sequel at all. GIP agonism is way less straightforward than GLP-1 agonism. GLP-1 agonism has a solid, easy-to-understand story. The GLP-1 receptors are activated when nutrients are consumed. Activating them has a lot of the effects of consuming lots of food: enhancing insulin secretion, reducing appetite, and increasing glucose metabolism. So, artificial long-acting GLP-1 agonists like semaglutide have, basically, the same effect on your body’s metabolism/satiety as consuming lots of food, but without the calories.
GIP agonism, by contrast, does not have an easy-to-understand story at all. GIP receptors, like GLP-1 receptors, are activated when food is consumed. However, the function of GIP is way harder to parse. It was first thought to decrease the secretion of stomach acid and gastric motility (hence, the name Gastric Inhibitory Peptide), but then that was found to only happen at supraphysiological levels (i.e. higher levels of the peptide than would be found in the body).
So then, the new story was that the function of GIP was to induce insulin secretion, just like the function of GLP-1 (hence, the new acronym Glucose-dependent Insulinotropic Peptide). Going along with this new role, GIP was also found to promote pancreatic beta cell proliferation, which are responsible for secreting insulin. All of this together should mean that GIP agonism works the same as GLP-1 agonism and makes people lose weight.
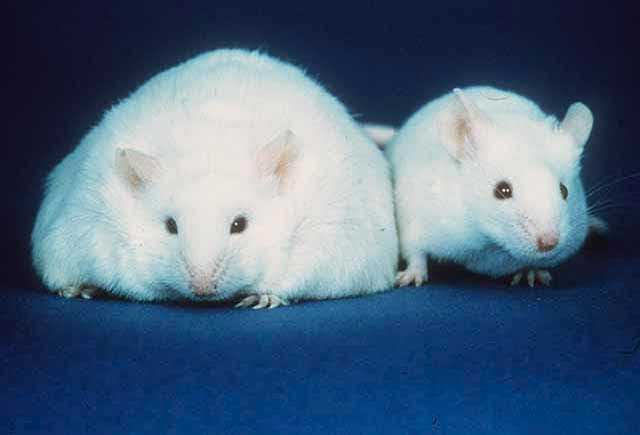
And it does. Sort of. But, again, GIP receptor agonism is way more complicated than this makes it sound. GIP also stimulates glucagon secretion, and glucagon, if you recall, is literally the opposite of insulin. And while, yes, while mice with chronically overexpressed GIP are protected from obesity, so are mice who are chronically deficient in GIP, including mice who don’t produce any GIP at all. Also, GIP on its own doesn’t seem to decrease body weight at all, but it does seem to have an additive effect when combined with GLP-1.
This isn’t just a mouse story, either. Amgen, a major pharmaceutical company, has announced that their new drug, a GLP-1 agonist/GIP inhibitor, shows safe, sustained, significant weight loss in obese humans. So, if they’re right, and the mouse studies are right, it seems like doing literally anything to GIP receptors (other than leaving them alone) while agonizing GLP-1 receptors results in greater weight loss than just agonizing GLP-1 receptors alone.
This is weird! Weight loss isn’t supposed to work this way. Our bodies are designed to maintain our weight and to add weight. It’s difficult to consistently lose weight, because for the vast, vast majority of our history as a species that was bad for your health. This is why it’s been so difficult for pharma companies to create safe, effective weight-loss drugs.
And so now we’ve got a receptor that seems designed to make us lose weight, safely, no matter what we do to it, and there doesn’t seem to be any physiological reason for it. There’s something funky here. For tirzepatide and other GLP-1/GIP dual agonists to actually be sequels to GLP-1 agonists, we have to understand what GIP’s function actually is. Otherwise, these drugs’ success in weight loss seems more like blind luck to me, and I wouldn’t be confident that future GLP-1 agonists wouldn’t work better than these dual agonists.
2. Does GLP-1 agonism cause people to lose muscle mass?
The second mystery is more straightforward. Some of the GLP-1 agonism trials have suggested that these drugs cause people to lose muscle mass. Some of them have not.
If these drugs do cause people to lose muscle mass, there is a lot of money to be made for whatever company successfully prevents the loss of muscle mass. Not only is loss of muscle mass an aesthetic concern, but it’s also a health concern, especially in the elderly. Loss of muscle mass is a major signal of imminent health problems in senior citizens, although admittedly the cause and effect is hard to parse out.
Now, how much money can be made from preventing loss of muscle mass is hard to say, as the total addressable market is somewhere between zero and all the people who are taking GLP-1 agonists, but it’s a large amount. And, again, it wouldn’t just be a bullshit innovation, as making GLP-1 agonists work better can be a big boon to health.
But, on the other hand, if GLP-1 receptor agonists don’t cause people to lose muscle mass, well, then, there’s not a lot to be done. The drugs don’t have any real problems then, besides the rebounding weight gain after people stop taking the drugs. This is an issue to be solved, but I think it will be by just making the drugs better.
Regeneron, Eli Lilly, and an assortment of startups are all betting on the idea that GLP-1 agonists cause muscle mass loss3. I think that’s an interesting mystery, and one that’s worth exploring.
So, that’s my pitch for the two questions I’ll be exploring in upcoming blog posts: what’s the deal with GIP, and what’s the deal with the supposed muscle mass loss. If that sounds interesting to you, stay tuned!
I swear I’m not doing all these size puns on purpose.
Unlike all the money that companies have gotten by basically legalizing psychedelics, like Johnson and Johnson with ketamine or everyone who’s trying to develop legal forms of LSD or psilocybin.
I mean, I get that it’s convenient, and even necessary, for doctors to have a form of ketamine that they can legally prescribe, and I get that there are patients who are legitimately helped by ketamine. But it’s just depressing (sorry) that the innovation there is just a regulatory innovation. Johnson and Johnson is making $500 million a year because they were able to jump through regulatory hoops to get nasal ketamine approved, but it seems unfair to me that the financial rewards go to J&J for their regulatory prowess, rather than anyone who was involved in the invention of ketamine or in its original application to depression.
Also, it’s worse for patients than if we just allowed them to snort ketamine, preferably at a German rave. Ah, well.
Interestingly, one well-funded longevity/AI startup, BioAge, bet on this muscle mass loss idea for like a year, trying to reposition their sarcopenia drug (that they bought from Amgen, funnily enough) as a drug for this and raising $170 million on that idea. Then, once Nature Biotechnology reported on it, they got spooked, and told Nature that actually they are only interested in pure weight loss. I have no idea why they did this and would love to know the actual story.