In my small, niche corner of Reddit, I recently went viral (by small, niche Reddit standards) for the following post.
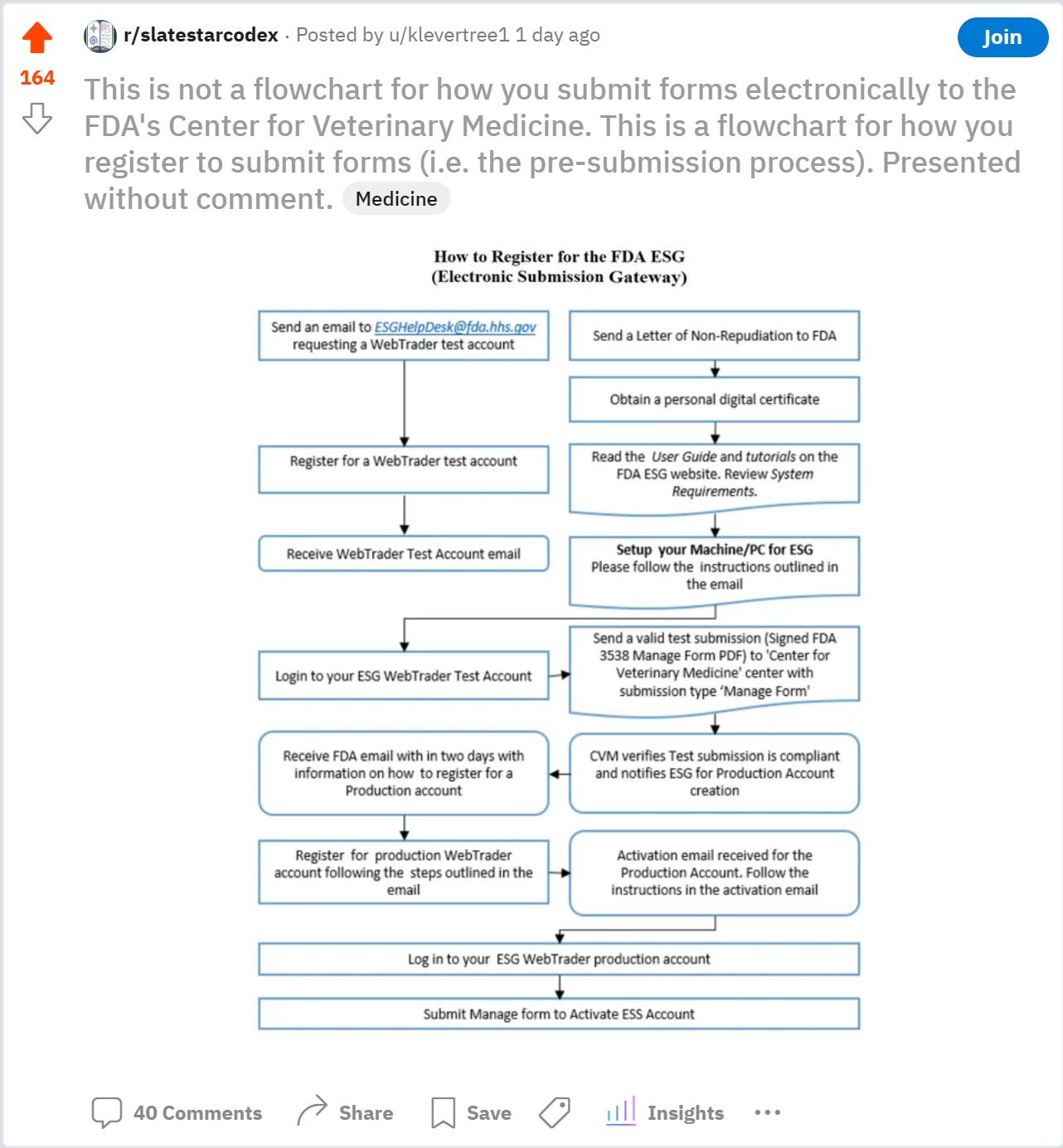
Someone in the comments said that “Presented without comment” is somewhat disingenuous, as I obviously did have a comment with the framing. Perhaps they were right. So, I thought I’d write my comment on this.
First of all, my interest in this is not academic. I have personally been struggling through this flowchart for about a month now, because I have to submit a document to get an exemption from the $130k/year fee for the FDA being aware of the fact that you are trying to get a drug approved (seriously). Note that this is separate from any of the fees you have to pay to actually get the drug approved.
The WebTrader software mentioned in the top left is so difficult to use that I actually hired a consultant to use it for me. The consultant got stuck because I needed to register with the FDA before he could register as a consultant for me, which neither of us knew. As it turns out, the “Letter of Non-Repudiation” is the registration.
Once I figured that out, I sent the letter. That was almost 3 weeks ago. Unfortunately, I found out yesterday that I sent the letter to the wrong address: the one mentioned on the website, rather than on the pdf that I got the flowchart from. So then I sent another letter to the right address, using the template in the pdf. However, once I sent the letter, I got suspicious that the template didn’t have a place to put my company name, which seemed weird for a registration letter. I called the FDA’s full-time technical help desk for electronic submissions, and he confirmed that I needed to modify the template to put my company’s name in.
I then sent another letter with the modified template. Hopefully, this one will be correct. From there, I will either use the consultant to use the WebTrader software for me, or I will once again figure it out myself, this time making full use of the FDA’s full-time technical help desk.
I think it’s important for me to note that this consultant is separate from the strategic consultant I have hired to help me deal with the FDA. While the strategic consultant helps me construct the letters that I send to the FDA, he is a retired veterinarian/pharm exec, which means that he does not feel comfortable giving technological advice on how to use the FDA’s electronic submission software. In fact, none of the strategic FDA consultants I talked to feel comfortable with that, and all of them encouraged me to hire another consultant for this technological issue.
The most absurd part of all of this, of course, is that this electronic submissions software was supposed to be the Center for Veterinary Medicine’s (CVM) great leap forward into the technological future, where this would be an easier and faster way of submitting documents than by mail. Well, actually maybe the most absurd part is that this flowchart is actually helpful, because I have been struggling for over a month to figure out the steps required to submit electronic documents to the CVM, and did not think to check on page 12 of a pdf on the FDA’s website. For the record, I have submitted electronic documents to multiple federal and state governmental agencies and have never had to go through something like this.
Actually, maybe the most absurd part is that, after all of this, I will still have to jump through all the hoops the CVM requires to submit all my documents in the proper format. This is only the beginning of my FDA hoop-jumping journey.
So, what are my overall thoughts on this?
1. This is an obviously ridiculous tax on productivity. Either I spend my time, my company’s money, or both trying to solve a problem that is entirely the FDA’s. Literally they could have an inbox that I email documents to, and templates as to what documents I need to email them. There aren’t that many companies working on veterinary drugs that the FDA needs a dedicated solution to electronic submissions, and certainly not one that’s this complex.
2. One of the most important parts of entrepreneurship, especially in hard-tech fields, is patience. Not to sound too self-congratulatory, but very few people can comfortably navigate being clever and willingly going through very dumb processes. I think a lot of very smart young people have great ideas but get stuck the first time they encounter something stupid they have to go through.
3. When I think about who benefits from this system, it seems pretty clear. There’s the FDA, who gets fewer submissions and gets to create a full-time help desk, whoever got paid to create this system, and the consultants.
Pharma suffers, but especially small or first time pharma (like my company), who don’t have dedicated people to navigate this. And then, of course, the consumer, who gets fewer pharma drugs and gets these costs passed onto them.
4. You may insert political commentary here.