As I’ve mentioned before in this blog, cavefish are fish that live solely in caves, as their name suggests. They are weird. They are obese, eyeless, and exceptionally long-lived. This is in contrast to their cousins, surfacefish, who live in rivers and streams near the caves and have normal sizes, normal eyes, and normal lifespans.
You may wonder why I call these fish “cavefish” and I never give their scientific names. That’s because they don’t have any. Cavefish is a generic term for any fish that lives solely in a cave, and represents a diverse group of over 200 fish, from catfish to eels.
Surfacefish can become cavefish rapidly in the right conditions, definitely within hundreds of years and likely within dozens. Surfacefish and cavefish can fully interbreed (as long as they’re part of the same original species), and surfacefish start the process of becoming cavefish in just a generation or two. In one interesting natural experiment, rainbow trout were introduced into a massive underground cave in Tennessee, Craighead Cavern, sometime around the 1950’s. The intention was to see where the cave exited by following the rainbow trout, but the trout stayed put, and have lived in the cave ever since. These rainbow trout today are pretty close to cavefish, even though they are constantly disturbed by tour guides tossing them food for the entertainment of tourists. Local news articles quote tour guides describing these rainbow trout as “enormous, 20% blind, and 70% colorless”, which, in my opinion, is a good description of being halfway in between cavefish and surfacefish.
This is not an adaptation that’s unique to fish, either. The olm is a species of aquatic salamander that lives almost exclusively in caves. Most olm are blind, with regressed eyes, and colorless (the exception to this description are a subspecies of olm which live in a spring near a cave, who have limited pigment and small eyes). Olm can survive at least 10 years without food, as they store enormous amounts of fat and glycogen in their liver. Their estimated lifespan is up to 110 years with a median of 58 years, far surpassing their nearest relatives in their family, mudpuppies, who only live about 11 years.
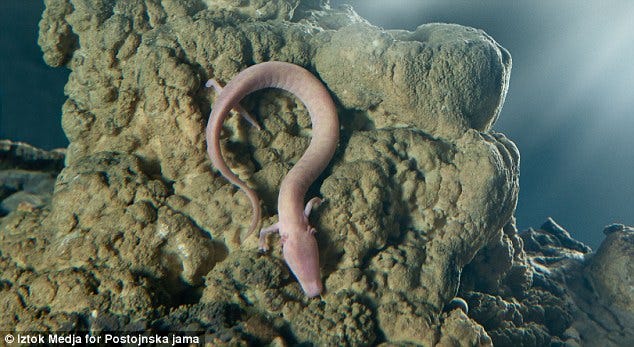
We are more closely related to olm than they are to rainbow trout, or to any of the other cavefish. And yet olm, when faced with cave conditions (darkness, lack of food, lack of predators), become remarkably phenotypically similar to fish in cave conditions. Most interestingly for me, their adaptations cause them to achieve two of the holy grails of human medicine: being obese without health consequences, and being exceptionally long-lived.
So, that raises the question: if you raised several generations of humans in a totally dark cave, would they, too, become obese, eyeless, and exceptionally long-lived, just like our cousins, the cave salamanders? And, if so, is there a way to trigger those changes without raising several generations of humans in total darkness? Is there a way to trigger just exceptional longevity without losing our eyes or slim figure? In other words, is being long-lived a choice?
It’s tricky to answer this because there hasn’t been enough research done on cavefish or olm yet. Researching longevity is, of course, difficult, because it takes a long time. There’s been more research done on the metabolic adaptations, but that’s also hard to measure without cutting open the animals and looking at their livers, which is generally a fatal procedure. So, in the end, we are unfortunately going to have to look at the least interesting of the adaptations because it’s the easiest to study: the loss of the eyes. We’ll then try to generalize to obesity and longevity.
So, the first question you might ask is: how do cavefish lose their eyes? Well, there are a few ways to answer that. The most obvious, and easiest to answer, is developmentally. Cavefish, at least the ones studied so far, lose their eyes in a famously strange way. As embryos (or “fry”), their eyes develop normally for a few days. Then all of a sudden, they start to lose their eyes, either lens first, in the case of the Mexican tetra, or retina first, in the case of the Somalian cavefish.
This roundabout way of getting rid of the eyes, by starting development on them and then erasing them, suggests that, for whatever reason, cavefish have to develop their eyes at least somewhat before erasing them. This is likely due to the sequencing of development in fish. Much like mammalian males develop nipples because sex differentiation happens after nipple formation, cavefish probably develop proto eyes because interfering earlier than that would mess up neurodevelopment.
If we zoom in on the Mexican tetra, who’s by far the best studied of the cave animals, we get a better idea of how exactly this sequencing of development works. If you look at a cave-dwelling Mexican tetra and a surface-dwelling Mexican tetra embryo develop side-by-side, you can notice that, from a very early stage, cave dwellers and surface-dwellers use the same cells for different purposes. They still form the same early structures, but cave-dwellers use differential timing in signaling genes to divert some retina cells to the hypothalamus. So, even though both cave dwellers and surface dwellers are working off almost the same blueprint for their construction project (similar enough DNA to interbreed), they have subtly different “foremen”. For the cave dwellers, this foreman can’t interfere with the blueprint, but he can redirect the materials as needed.
Even more interesting, we also have an excellent idea of how the eye eventually self-destructs. Remember how I said the Mexican tetra’s eye destruction starts lens first? Well, in a fascinating series of experiments, it was found that transplanting a lens from a surfacefish tetra embryo to a cavefish embryo results in the cavefish developing a normal eye on the side of the transplant. Likewise, transplanting a lens from a cavefish to a surfacefish results in the surfacefish developing an eye that self-destructs on the side of the transplant.
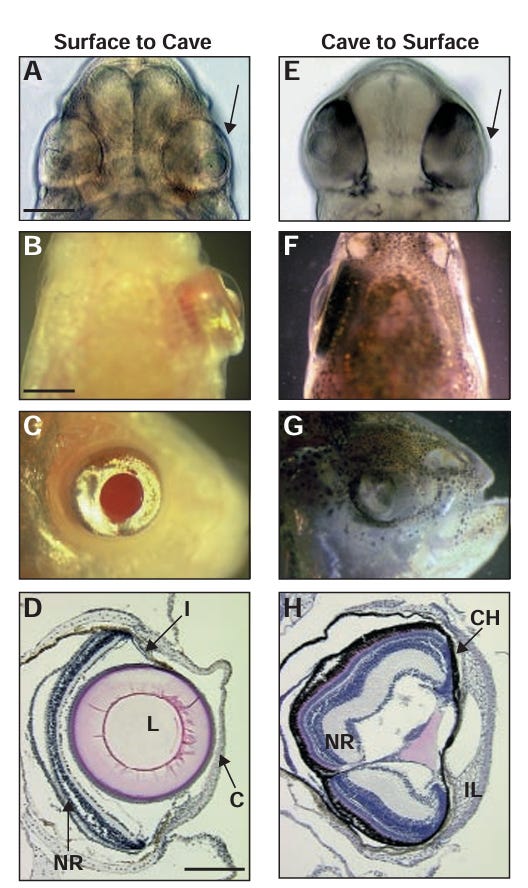
So, for the Mexican tetra at least, the signal to keep the eye or not keep the eye, and likely the signal to divert cells or not divert cells from the retina, comes directly from the lens. It is not a body-wide signal. Now, the question becomes: is the signal genetic or epigenetic? That is: does the signal come directly from permanent modifications to the genetic code, or does the signal come from temporary modifications to how the genetic code is read?
The implications of either conclusion are important. If these dramatic phenotypic changes are genetic, then they have to be already harbored in the cavedweller’s genetic code as a silent, or cryptic, variant. There’s no way that random mutations can come about in just a few generations so as to cause such dramatic phenotypic changes, especially consistently across cavedwelling species. So, instead, all cavedwelling species have to already have these phenotypes within their genome, including the phenotype to become long-lived. Under pressure of natural selection, these phenotypes get suddenly unleashed.
On the other hand, if these dramatic phenotypic changes are epigenetic, then these are massive, heritable phenotypic changes that can be triggered by a simple change in conditions. By simply silencing one region of the genome or promoting another in response to a change in conditions, cavedwelling organisms can become the blind, obese, longlived cavedwellers we know and love. And, somehow, all the cavedwellers adopt similar phenotypes, despite having epigenetic changes targeted at different parts of the body, like with our retina vs. lens directed eye apoptosis.
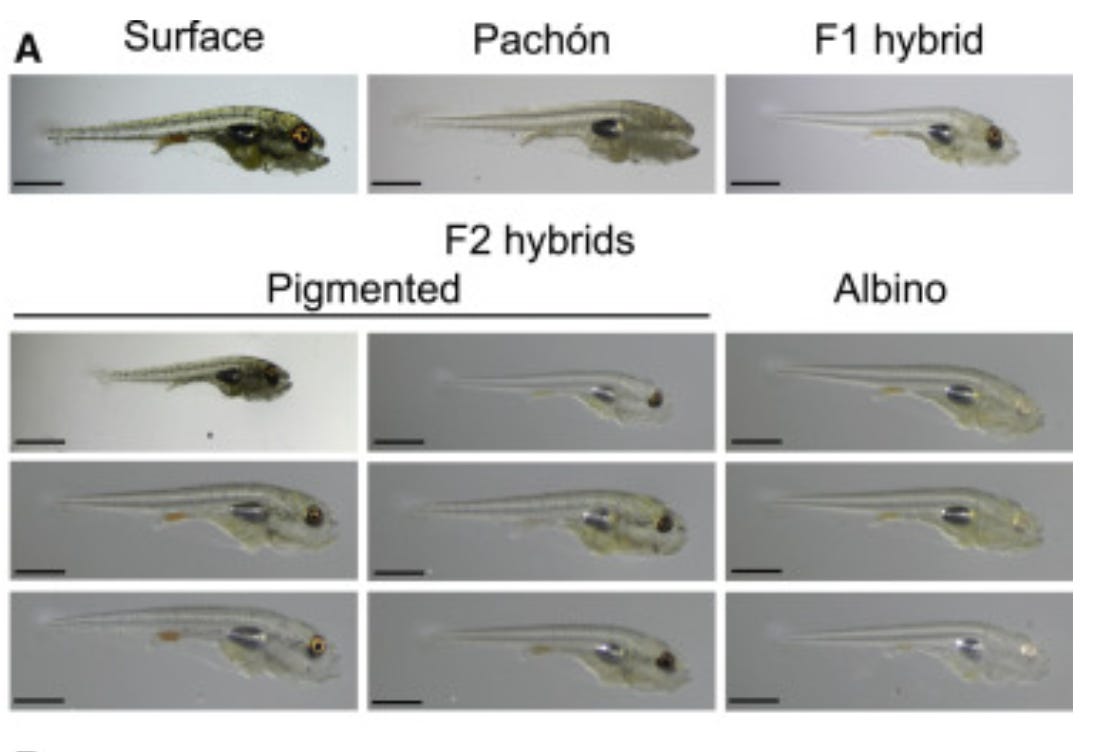
Now, the jury is still out on which one of these mechanisms is responsible, but the evidence leans towards the former, the cryptic variation. The cavefish phenotype is conserved, in that, if you breed cavefish with each other, they will continue to be eyeless. And, if you cross a cavefish with a surfacefish, the offspring have small eyes, with bigger eyes as you continue to cross. This is identical to a classic Mendelian recessive genotype experiment. Meanwhile, it’s rare for epigenetic inheritance to function across generations, and probably impossible for it to mimic Mendelian recessive genotyping.
Even stronger evidence comes from gene editing, where a CRISPR-induced mutation in a single gene, oca2, can cause both albinism and reduced sleep needs in the Mexican tetra, two of the less commonly discussed cavefish adaptations. Unfortunately, there’s no similar simple CRISPR-induced mutation that can cause complete eye loss, but it raises the likelihood that there is one, or at least a few that function together to cause eye loss.
But the trouble with cryptic variation is that it’s, well, cryptic. It’s hard to understand the mechanism. Normal variation is pretty easy to understand, like the classic peppered moth example. Before the Industrial Revolution, most moths were white, and a few were black. After the Industrial Revolution, black moths were better able to hide amongst the coal covered trees, and so were better able to reproduce before being eaten by birds. The frequency of black moths rose drastically.
This sort of variation does not exist for the phenotype of having eyes. When surfacefish population get trapped in a cave, all of them have eyes, with presumably only minute variation in sizing. The step change to losing eyes in development (and becoming obese, etc.) can’t be driven by just better reproductive success. So, something else has to trigger it.
The best guess so far is that it’s triggered by the lack of conductivity in cavewater, as cavewater contains much fewer solutes than freshwater. This lack of conductivity elicits a “heat shock” response, forcing the cavefish to use up their heat shock protein 90 (hsp90) in order to keep normal protein folding in this strange, nonconductive environment. In experimentation, inhibiting the hsp90 protein with radicicol results in a massive increase in variation of tetra eye sizes, from very big to very small, which would then, presumably, allow for the same selection mechanism as the peppered moths.
If we zoom out again, then we get a sense of how obesity or longevity could be triggered in cavefish or olm or, indeed, humans. Being raised in a dark, food desert of a cave is a stressor. Whatever buffer systems are involved in managing stress of various sorts, whether hsp90, cortisol or something else goes into overdrive trying to manage the stress. The buffer system runs thin quickly, as metabolic differences between dark-raised surfacefish and light-raised surfacefish can be seen in as little as 2.5 days.
As the buffer system runs thin (i.e. cortisol or hsp90 runs out), extreme phenotypic variations are unearthed. In humans, we’d call this a disease, like Addison’s disease. In reality, it’s an evolutionary adaptation to extreme conditions. If the phenotype provides an advantage, like the loss of eyes providing an energy saving advantage that makes up for the loss of eyesight, then that phenotype will win out.
The key here, though, is that those genetic variants already exist. They’re just masked. The tetra that go into the caves already have, in their genetic code, extreme variations in eye size, obesity, and longevity. However, the buffer systems on top of the genetic code smooth out all the variations. Without the buffer system, you would see surfacefish with tiny, almost nonexistent eyes and surfacefish with huge eyes. With the buffer system, those same fish get just slightly smaller and slightly bigger eyes.
If this is true for tetra, which, to be fair, is still an if, then it might be true for olm as well. And if it’s true for olm, then it might be true for humans. That means that, within our genetic code, there could be extreme variations in body weight or longevity that are naturally masked by buffer systems. If we inhibit or exhaust the buffer systems, then those natural, extreme variations would reveal themselves.
This, if true, would be neat. Really neat. Unfortunately, as far as I can tell, there’s no good way to test this, because there aren’t any mammal species who live full time in caves or even full time in complete darkness. There definitely isn’t the natural experiment of two subspecies who can interbreed, one in darkness and one in light, like there is for surfacefish and cavefish.
That being said, both bats and moles live almost all the time in darkness or very low light conditions, and they do both have generally poor eyesight and generally long lives. Some bats and some moles are exceptional on either measure, with one tiny bat species living up to 41 years and one mole species, the naked mole rat, living up to 37 years. And, for naked mole rats at least, this longevity comes hand in hand with a very low metabolic rate during hypoxic conditions, an intriguing parallel to the low metabolic rate accompanying the obesity of olms and cavefish.

And, if I were to really go out on a limb with my free association, I’d point out that mice, when born and raised in complete darkness, have both pronounced retinal changes and “depressive” symptoms that persist after return to the light, which seems close to a low metabolic rate. They don’t have any consistent changes in bodyweight or longevity, but still, you know, there’s something here if you squint at it, no pun intended.
But I’ll let these sleeping fish, and mice, lie for now. Until someone figures out a method and the consequences of exhausting buffer systems in mammals, and exploits that to develop mammals with extreme phenotypes of longevity or obesity (and to identify the cryptic gene variants that hide those phenotypes), there’s not a lot for us to do. Longevity still might be a choice, but, unfortunately, we’re still in the dark about it.